History
Originally established in 1975 as the Veterinary Infectious Disease Organization, we have evolved from a small agricultural focused research organization to a world-class research institute dedicated to the development of vaccines for the protection of human and animal health.
1975

Saving Livestock through Research
The Veterinary Infectious Disease Organization (VIDO) was founded with financial support from the Devonian Group of Charitable Foundations, the Provinces of Saskatchewan and Alberta and the University of Saskatchewan. Pictured, VIDO's founding Director Dr. Christopher Bigland.
1978

Official Opening
VIDO officially opened its permanent laboratory and animal isolation facilities.
1978

First Vaccine Launched
VICOGEN™, an E. Coli scours vaccine for cattle, was launched in collaboration with Connaught Laboratories.
1983

Launch of BIOSTAR Inc.
BIOSTAR Inc., a federally incorporated company was launched to market animal health technologies and products developed at VIDO to national and international biological production companies.
1983

Second Director Appointed
Dr. Stephen Acres replaces Dr. Chris Bigland as VIDO’s second Director, with Dr. Lorne Babiuk appointed as the Director of Research.
1984

Second Vaccine Launched
Commercialization of ECOLAN RC™, a subunit vaccine against calf scours caused by E. coli, rotavirus and coronavirus.
1986
New 'World-First' Vaccine
Launch of HEVLAN TC™, the world’s first vaccine for hemorrhagic enteritis of turkeys.
1987

Research Station Opens
VIDO opens its 160 acre research station for large scale animal trials.
1992
3 New Vaccines Launched
Three new vaccines for bovine respiratory diseases commercialized: Pneumo-Star™, Somnu-Star™ and Somnu-Star Ph.™
1993

Third Director Appointed
Dr. Lorne Babiuk becomes VIDO’s third Director.
1997

Swine Vaccine Commercialized
Commercialization of Pleuro-Star 4™, a vaccine for swine that stimulates a protective immune response against the common strains of the bacteria causing swine pleuro-pneumonia.
2003

Containment Level 2 Expansion Opens
VIDO opens a new $17.8 million, 50,000 square foot expansion of containment level 2 laboratories and offices. Funding received from the Canadian Foundation for Innovation, the Province of Saskatchewan and the Province of Alberta.
2003

New Name and Logo
Renamed as the Vaccine and Infectious Disease Organization to reflect expanded research goals.
2007

Fourth Director Appointed
Dr. Andrew Potter takes over as VIDO’s new Director & CEO.
2008

Econiche Launch
Econiche™, a vaccine for cattle against E. coli O157:H7, co-developed by researchers at the University of British Columbia commercialized by Bioniche Life Sciences Inc..
2008

PREVENT Established
VIDO-InterVac, the Canadian Center for Vaccinology, and the BC Centre for Disease Control fund the establishment of the Pan-Provincial Vaccine Enterprise Inc., a not-for-profit organization focused on commercializing promising human vaccine candidates.
2011

InterVac Grand Opening
VIDO celebrates the opening of its $140 million containment level 3 facility, the International Vaccine Centre (InterVac). The event was attended by Prime Minister Stephen Harper, Saskatchewan Premier Brad Wall and Saskatoon Mayor Donald Atchison.
2013

InterVac Goes Hot
Containment level 3 projects begin following final certification from government agencies.
2013

ISO 9001 Certification
VIDO's Management System is certified to ISO 9001.
2019

Fifth Director Appointed
Dr. Volker Gerdts selected as VIDO's fifth Director and CEO.
2020

VIDO first to isolate SARS-CoV-2 in Canada
VIDO became the first laboratory in Canada to isolate SARS-CoV-2 from a clinical sample obtained from Sunnybrook through the Public Health Agency of Canada. The isolated virus was shared with federal and provincial laboratories.
2020

First Animal Model for SARS-CoV-2
VIDO was the first lab in Canada to develop an animal model (ferret) against SARS-CoV-2. The model is vital in the development of vaccines, antivirals, and therapeutics and was widely used by government, industry and academic partners worldwide.
2020
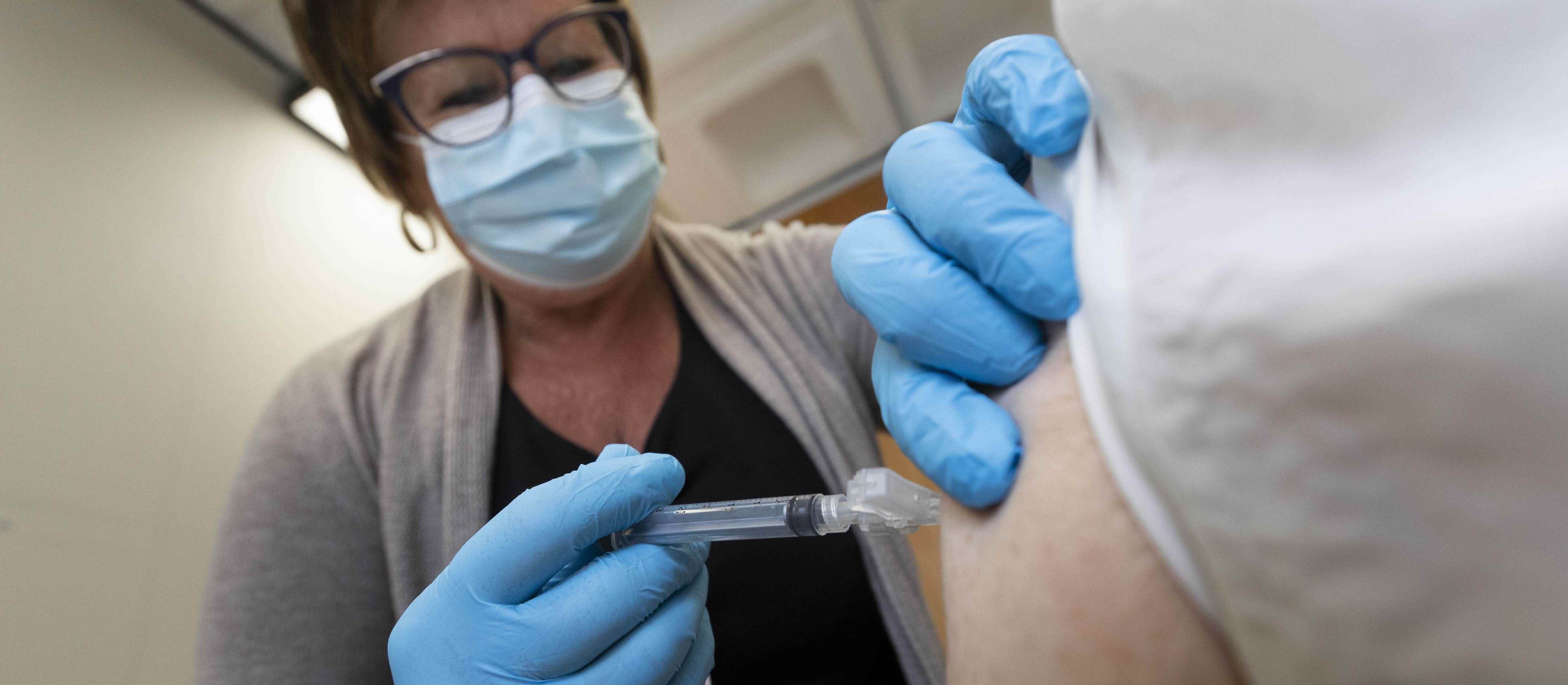
First Canadian University to have a SARS-CoV-2 Vaccine in Clinical Trials
VIDO/USask became the first university in Canada to have a vaccine (COVAC-2) in clinical trials for SARS-CoV-2. VIDO’s vaccine produced was safe and produced a strong immune response in humans and went on to Phase 2 clinical trials.
2021

New Visual Identity
VIDO embraced a new logo that symbolizes our national and international impact.
2022

Vaccine Development Centre (VDC) Opens
The VDC opened with manufacturing capabilities for human and animal vaccines, strengthening Canada’s capacity to respond to future pandemics.
2024

Construction Begins on New Animal Facility
Construction started on a new animal housing facility designed to support infectious disease research. The facility will be equipped to meet evolving research needs while upholding high standards of animal care and ethics.
2025

Construction Begins on CL4 expansion
Renovations began to add CL4 capacity — the highest level of biocontainment — enabling research on any pathogen, including those that pose the greatest risk to global health.

